H2o Lewis Structure

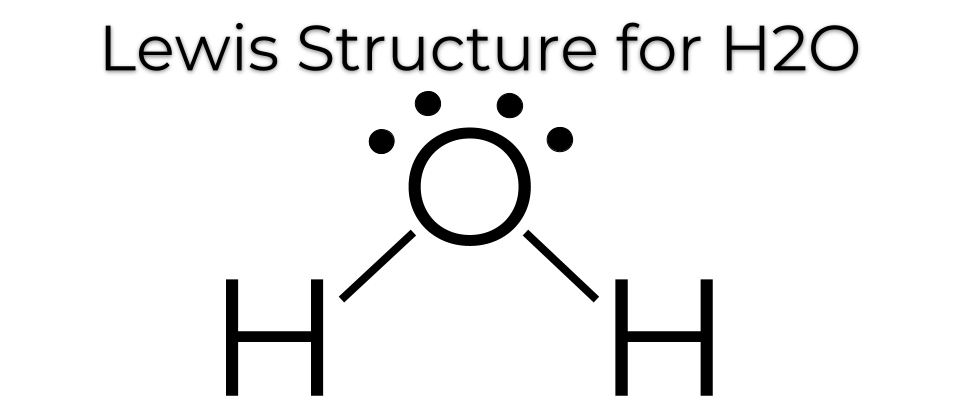
The Lewis Dot Structure For H2o

File H2o Lewis Structure Png Png Wikimedia Commons

Water Lewis Structure How To Draw The Lewis Structure For Water Youtube
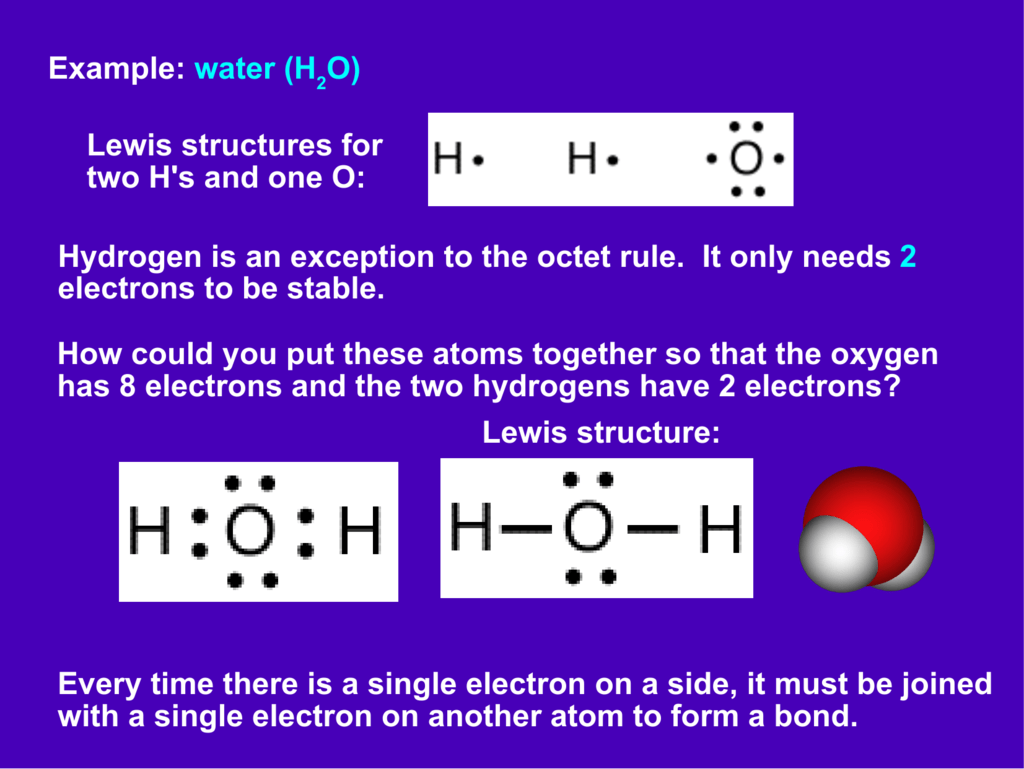
H2o lewis structure
Lets do the lewis structure for water. How to draw lewis dot structures duration. Whenever we are drawing a lewis structure the first thing we have to do is determine the number of valance electrons we have for our structure. This increases electron electron repulsion and therefore creates a bent structure as opposed to co2s linear structurethis bent molecular structure gives it many unique properties such as being polarone of the most fascinating phenomena is the idea of hydrogen bonding. Draw the lewis structure for h20. There is an atom of oxygen in the center and two atoms of hydrogen around the central atom. Drawing the lewis structure for h 2 o. 2 plus 6 equals 8. H2os lewis dot structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. There are also two pairs of electrons around the oxygen which you can see at the lewis structure. But we have two of them so lets multiply that by 2. N 2 o lewis structure resonance structures oxidation number n 2 o nitrous oxide is an oxide of nitrogen and is called as laughing gas. And for oxygen we have six electrons. In order to determine the molecular geometry for h2o observe the lewis structure of the same. Another straight forward lewis structure.
And oxygen is in group 6 sometimes called 16 so it has 6 valence electrons. Lewis diagrams made easy. So 1 times 2 is 2 plus 6. Wayne breslyn 151634 views. There is an easy way and a formal way to draw the lewis structure of h 2 o water. We have a total of eight valence electrons. Water is probably one of the most frequently taught and arguably important lewis structures use to introduce the topic of lewis structures. How to draw the lewis structure for n 2 o drawing. You have a total of 8 valence electrons available to fill the octets of oxygen and hydrogen. Remember that hydrogen only needs two electrons to have a full outer shell. On the periodic table hydrogens in group 1 it has 1 valence electron. In the formal way we find how many electrons we have step 1 how many each atom needs step 2 how many of those are bonding step 3 4 and how many are lone pairs step 5. Water lewis structure how to draw the lewis structure for water duration. Try to draw the h 2 o lewis structure before watching the video. H2o lewis structure ure resonance structures oxidation number.
Related post:
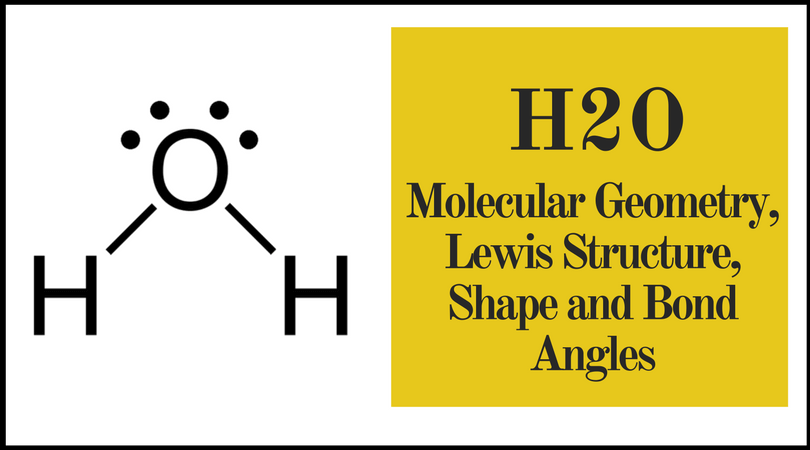
H2o Molecular Geometry Lewis Structure Shape And Bond Angles

What Is The Electron Dot Structure Of H20 Quora

H2o Lewis Structure W A Free Video Guide

Lewis Dot Structure For H2o Transparent Cartoon Free Cliparts Silhouettes Netclipart

Water Lewis Structure How To Draw The Lewis Structure For Water Youtube

H2o Structure Model Https Ift Tt 2qs2adi In 2020 Molecular Geometry Molecule Model Air Gear Anime

H2o Lewis And 3 D Structure Dr Sundin Uw Platteville
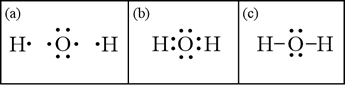
What Is The Lewis Structure Of H2o Socratic
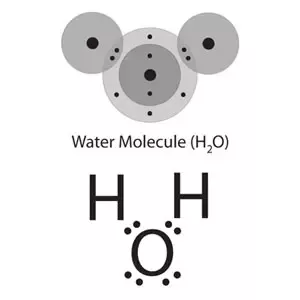
How To Find The Electron Dot Diagram For H2o Quora

Hydrogen Bonding H2o Molecular Structure Clipart 502646 Pinclipart

H2o Molecular Geometry Shape And Bond Angle Precise Angle Is 104 45 Youtube
Https Oneclass Com Homework Help Chemistry 17352 How Can I Draw The Lewis Struct En Html

Reduction Of O2 To H2o And Its Free Radical Intermediates A Lewis Download Scientific Diagram
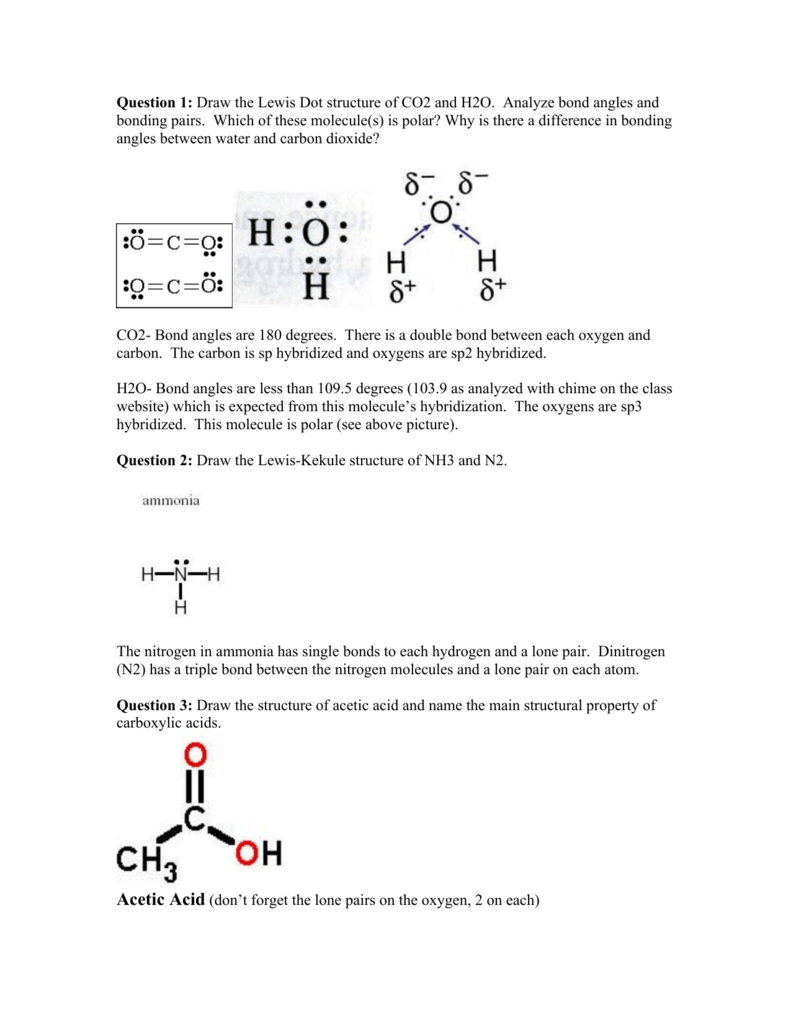
Question 1 Draw The Lewis Dot Structure Of Co2 And H2o Analyze
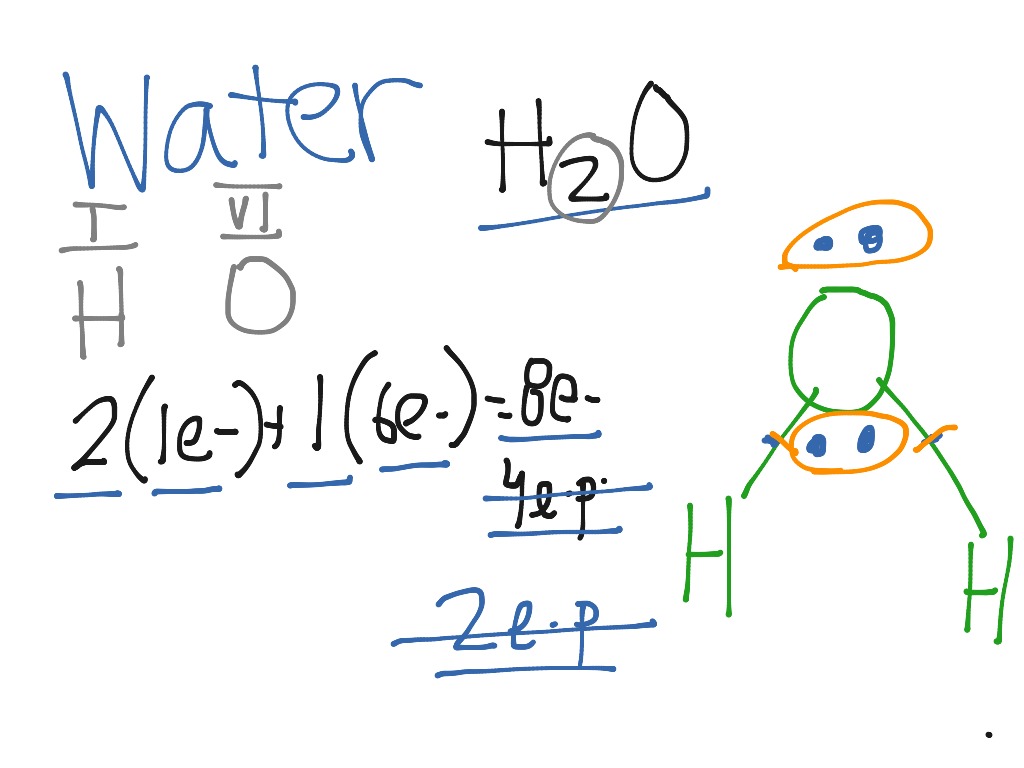
Water H2o Lewis Dot Structure Science Chemistry Lewis Dot Diagram Showme
What Is The Lewis Structure Of H2o Quora
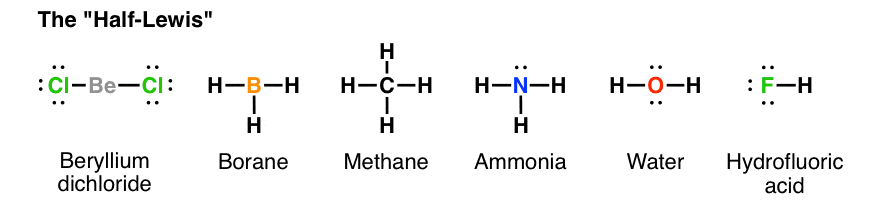
From Gen Chem To Org Chem Pt 7 Lewis Structures Master Organic Chemistry

Draw The Lewis Structure Of H2o Include Any Nonbonding Electron Pairs Draw The Molecule By Placing Brainly Com
That's all about H2o lewis structure, H2o lewis structure ure resonance structures oxidation number. Try to draw the h 2 o lewis structure before watching the video. Water lewis structure how to draw the lewis structure for water duration. In the formal way we find how many electrons we have step 1 how many each atom needs step 2 how many of those are bonding step 3 4 and how many are lone pairs step 5. On the periodic table hydrogens in group 1 it has 1 valence electron. Remember that hydrogen only needs two electrons to have a full outer shell.

